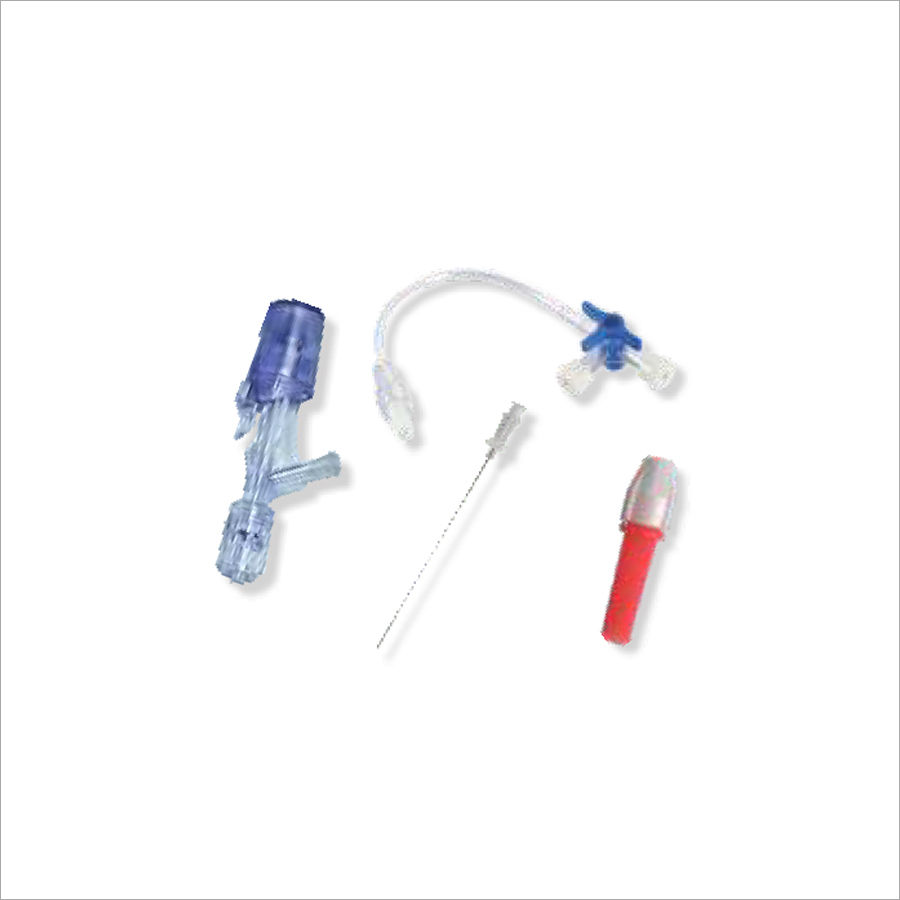
PTCA Y-Connector Push Click System
PTCA Y-Click Connector, provides an easy -touse hemostatic device for both diagnostic and interventional procedures. This ergonomically designed device significantly reduces the blood loss during interventional procedures. Among the Y-Click most unique advantages are its self closing mechanism and semi -open position, as well as its one - handed operation.
PTCA Y-Connector Push Pull System
PTCA Y-Push Pull Connector, which is used for coronary intervention, Push Pull head knob, which ensures minimal blood and precise navigation of devices in the vasculature. A specially engineered valve with micro vertical slits which facilitate smooth manipulation of device nullify blood loss.
PTCA Y-Connector Screw Type System
Touhy Borst also know a Screw Type Y Connector, which is used for coronary intervention. It has a latest screw type head knob, which ensures minimal blood adjustable Thumb Wheel Mechanism pervents over tightening, loss of bloods and devices movement when turned. Allow for insertion of different size devices.
| Type | Description | Ref. Code |
| PTCA Y-PUSH CLICK SET | Hemostasis Y Connector Push Click System With Extension Line « Blunt Needle * Torque Device | MTPYK-PCO1 |
| PTCA Y-PUSH PULL SET | Hemostasis Y Connector Push Pull Type With Extension Line * Blunt Needle « Torque Device | MTPYK-PP01 |
| PTCA SCREW TYPE SET | Hemostasis Y Connector Screw Type With Extension Line * Blunt Needle * Torque Device | MTPYK-ST01 |
The PTCA Y Connectors advanced rotating hemostatic valve ensures a tight, leak-resistant seal, even under high pressure. The ergonomic, one-hand operation promotes efficient workflow, while the clear, transparent housing allows for optimal visual monitoring during critical interventional procedures. Each kit includes essential accessories like a torque device, Tuohy borst adapter, and guidewire introducer for comprehensive usability.
Precision and Compatibility in CardiologyExpertly engineered for compatibility with standard PTCA guidewires and catheters, these kits support accurate device manipulation. Manufactured from medical-grade materials, they assure patient safety and device reliability. The high sealing accuracy is matched with secure luer lock fittings, making the kits especially beneficial for interventional cardiologists and catheterization labs.
Sterility and Compliance You Can TrustEvery PTCA Y Connector & Kit is EO sterilized, completely latex-free, and DEHP-free to meet stringent healthcare standards. With ISO 13485 certification, each unit undergoes rigorous quality checks, ensuring consistent performance. The products are supplied in individually wrapped, sterile pouches, providing peace of mind for both user and patient.
FAQs of PTCA Y Connector & Kits:
Q: How do I use the PTCA Y Connector & Kits during a PTCA procedure?
A: Begin by ensuring all kit components remain sterile before use. Attach the Y-Connector to the catheter using the luer lock fitting for a secure connection. Guidewire introducers and the torque device assist in precise guidewire manipulation, while the rotating hemostatic valve helps maintain a leak-proof seal during injections or monitoring.
Q: What are the key benefits of using this Y-Connector with rotating hemostatic valve?
A: The connector provides leak-proof security, one-handed ergonomic handling, and superior control over catheter and guidewire movements. Its transparent design enhances visual monitoring, while the high-pressure resistance (up to 400 psi) maintains safety and performance throughout the procedure.
Q: When should I consider using this PTCA Y Connector & Kit?
A: These kits are specifically designed for use during PTCA (Percutaneous Transluminal Coronary Angioplasty) procedures, particularly when precise control and secure, sterile access for catheter and guidewire manipulation are required.
Q: Where is this product commonly utilized?
A: The PTCA Y Connector & Kits are primarily used in cardiac catheterization laboratories and interventional cardiology departments where PTCA and similar minimally invasive procedures are performed.
Q: What is the process for ensuring compatibility with my existing equipment?
A: The kits are compatible with standard PTCA guidewires and catheters. Simply check that your equipment uses typical dimensions, as the connectors use standard luer lock fittings and industry-accepted sizes.
Q: How is sterility and patient safety maintained for this product?
A: Each kit is EO (ethylene oxide) sterilized, individually packed in a sterile pouch, and produced with latex-free, DEHP-free materials. These factors, alongside ISO 13485 certification, ensure the highest levels of patient safety and infection prevention.
Q: What shelf life and storage guidelines should I follow?
A: These kits have a shelf life of 5 years. Store them in a cool, dry place away from direct sunlight to maintain sterility and product integrity.