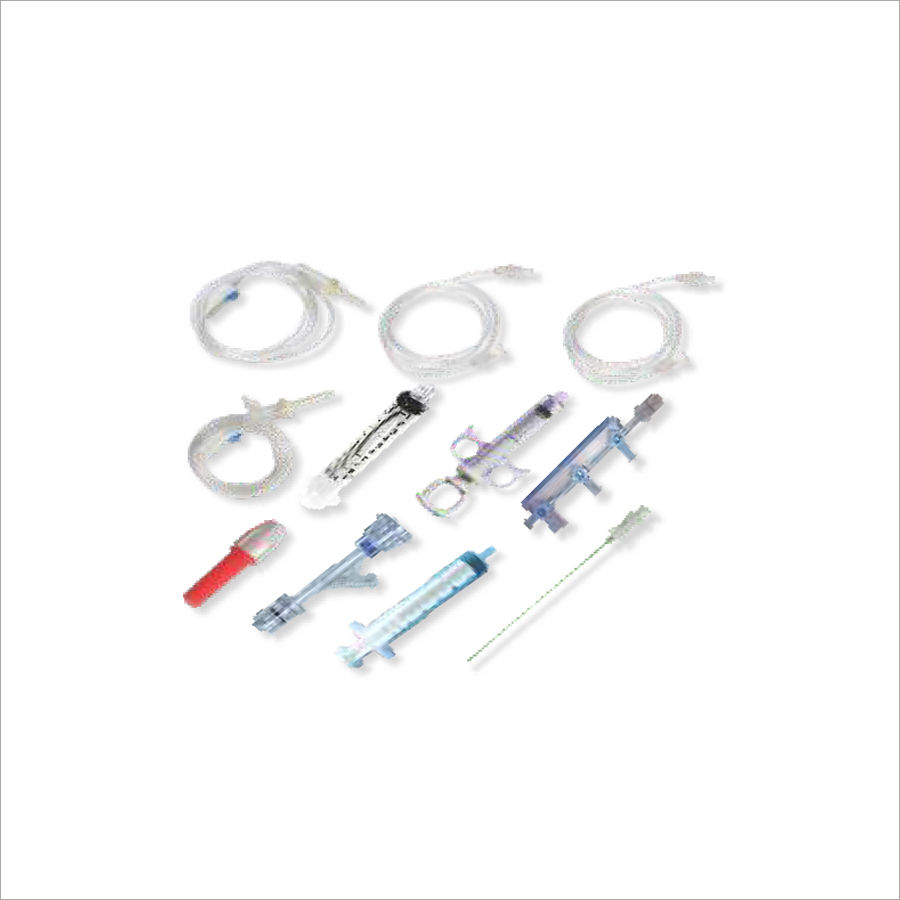
Medtech Devices PTCA Kit is a customized kit for use in angioplasty.
PTCA Kit is accessible with a minimally invasive procedure so as to open up as well as blocked coronary arteries. It enables the blood to circulate in an unobstructed way to the heart muscle. This is a complete made-to-order package of several medical equipment needed for the purpose of angioplasty. It has been made up of medical grade material of high quality. PTCA Kit makes reduction in the setup time as well as total procedure cost. It can also reduce the packaging and ensures saving the valuable space.
Specialized kit ensures safety and convenience for surgeons and physicians while doing the procedure.
- Manifold 3 Port Right-on/off » Hemostatic Y Connector Screw type
- Blunt Needle - Control Syringe 12 ml +» Syringe 10 ml Normal
- Syringe 10 ml Luerlock » Torque «H.P.T.50cm
- P.M. Line 200 cm « IV Set Vented + IV Set Non Vented
Designed to work seamlessly with all standard PCI guiding catheters, the PTCA Kit enables flexible integration into diverse cardiovascular intervention procedures. Its radiopaque markers enhance visibility under fluoroscopy, ensuring precise placement during angioplasty. This compatibility maximizes procedural efficiency and safety for clinicians.
Sterile and Patient-Safe DesignSterilized using Ethylene Oxide (ETO), the PTCA Kit ensures a high standard of patient safety. It is latex-free and CE marked, minimizing allergy risks and assuring compliance with international standards. The single-use, disposable nature reduces infection risk while maintaining optimal function throughout the procedure.
Convenient Packaging and Complete ContentsEach kit is supplied in a secure blister pack with a peelable sterile pouch, preserving sterility until use. The kit includes all essential components for angioplasty: guide wire, inflation device, Y-connector, torque device, syringes, introducer sheath, and needles. Clear instructions for use accompany every kit, supporting user confidence and ease of operation.
FAQ's of PTCA Kit:
Q: How is the PTCA Kit used during a coronary angioplasty procedure?
A: The PTCA Kit is used to facilitate balloon angioplasty by providing all essential tools-including guide wires, introducer needles, syringes, and dilators. Once the vascular access is established, the components work in tandem to enable precise catheter insertion, inflation, and angioplasty in coronary or peripheral vessels.
Q: What are the benefits of the PTCA Kit's radiopaque markers?
A: Radiopaque markers improve visibility under fluoroscopy, allowing clinicians to accurately position and monitor the catheter and other components during angioplasty procedures. This ensures high procedural precision and enhances patient safety.
Q: When should the PTCA Kit be replaced or disposed of?
A: The PTCA Kit is intended for single-use only and must be disposed of immediately after the procedure to maintain sterility and prevent cross-contamination. Always check the expiry date indicated on the packaging before use.
Q: Where can the PTCA Kit be stored to maintain its shelf life?
A: Store the PTCA Kit in a cool, dry environment away from direct sunlight. Proper storage preserves its sterility and extends its shelf life to up to 5 years from the date of manufacture.
Q: What process is involved in preparing the PTCA Kit for use?
A: Open the blister pack and peelable sterile pouch using aseptic techniques. Review the included instructions for use and prepare all components on a sterile field prior to patient contact, ensuring all items are intact and within expiry.
Q: How does the PTCA Kit benefit healthcare providers and patients?
A: The kit streamlines angioplasty procedures by providing all necessary, pre-sterilized components, minimizing preparation time and reducing the risk of infection. Its immediate availability and high-precision design lead to improved outcomes for both clinicians and patients.